Khan Academy on a Stick
Acids and bases
-
 Acid Base Introduction
cc
Acid Base Introduction
ccArrhenius, Bronsted Lowry, and Lewis Acids and Bases.
-
 pH, pOH of Strong Acids and Bases
cc
pH, pOH of Strong Acids and Bases
ccCalculating the pH or pOH of strong acids and bases.
-
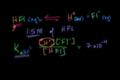 pH of a Weak Acid
cc
pH of a Weak Acid
ccCalculating the pH of a weak acid
-
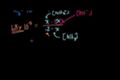 pH of a Weak Base
cc
pH of a Weak Base
ccpH of .2 M of NH3 (weak base).
-
 Conjugate Acids and Bases
cc
Conjugate Acids and Bases
ccIntroduction to conjugate acids and bases
-
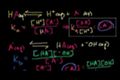 pKa and pKb Relationship
cc
pKa and pKb Relationship
ccThe pKa and pKb relationship between conjugate acids and bases (both of which are weak).
-
 Buffers and Hendersen-Hasselbalch
cc
Buffers and Hendersen-Hasselbalch
ccBuffers and the Hendersen-Hasselbalch equation
-
 Strong Acid Titration
cc
Strong Acid Titration
ccStrong acid titration and equivalence point
-
 Weak Acid Titration
cc
Weak Acid Titration
ccEquivalence point when titrating a weak acid
-
 Half Equivalence Point
cc
Half Equivalence Point
ccFiguring out the pKa of an unknown weak acid from the half equivalence point
-
 Titration Roundup
cc
Titration Roundup
ccMaking sure you fully understand titration curves
-
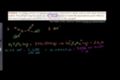 Acid Base Titration
cc
Acid Base Titration
ccUsing acid-base titration to find mass of oxalic acid