Khan Academy on a Stick
Thermodynamics
-
 Thermodynamics (part 1)
cc
Thermodynamics (part 1)
ccIntuition of how gases generate pressure in a container and why pressure x volume is proportional to the combined kinetic energy of the molecules in the volume.
-
 Thermodynamics (part 2)
cc
Thermodynamics (part 2)
ccExample problem that pv=pv. Introduction to temperature.
-
 Thermodynamics (part 3)
cc
Thermodynamics (part 3)
ccIntroduction to Kelvin. Example of a problem involving the ideal gas law
-
 Thermodynamics (part 4)
cc
Thermodynamics (part 4)
ccIntroduction to the concept of a mole. Universal gas constant R. PV=nRT
-
 Thermodynamics (part 5)
cc
Thermodynamics (part 5)
ccExample problem involving PV=nRT
-
 Macrostates and Microstates
cc
Macrostates and Microstates
ccThe difference between macrostates and microstates. Thermodynamic equilibrium.
-
 Quasistatic and Reversible Processes
cc
Quasistatic and Reversible Processes
ccUsing theoretically quasi-static and/or reversible processes to stay pretty much at equilibrium.
-
 First Law of Thermodynamics/ Internal Energy
cc
First Law of Thermodynamics/ Internal Energy
ccFirst law of thermodynamic and Internal Energy
-
 More on Internal Energy
cc
More on Internal Energy
ccGetting more intuition of internal energy, heat, and work
-
 Work from Expansion
cc
Work from Expansion
ccHow a system can do work by expanding
-
 PV-diagrams and Expansion Work
cc
PV-diagrams and Expansion Work
ccWhy work from expansion is the area under the curve of a PV-diagram
-
 Proof: U=(3/2)PV or U=(3/2)nRT
cc
Proof: U=(3/2)PV or U=(3/2)nRT
ccConceptual proof that the internal energy of an ideal gas system is 3/2 PV.
-
 Work Done by Isothermic Process
cc
Work Done by Isothermic Process
ccIsothermic and Adiabatic processes. Calculating the work done by an isothermic process. Seeing that it is the same as the heat added.
-
 Carnot Cycle and Carnot Engine
cc
Carnot Cycle and Carnot Engine
ccIntroduction to the Carnot Cycle and Carnot Heat Engine
-
 Proof: Volume Ratios in a Carnot Cycle
cc
Proof: Volume Ratios in a Carnot Cycle
ccProof of the volume ratios in a Carnot Cycle
-
 Proof: S (or Entropy) is a valid state variable
cc
Proof: S (or Entropy) is a valid state variable
ccProof that S (or entropy) is a valid state variable.
-
 Thermodynamic Entropy Definition Clarification
cc
Thermodynamic Entropy Definition Clarification
ccClarifying that the thermodynamic definition of Entropy requires a reversible system.
-
 Reconciling Thermodynamic and State Definitions of Entropy
cc
Reconciling Thermodynamic and State Definitions of Entropy
ccLong video explaining why entropy is a measure of the number of states a system can take on (mathy, but mind-blowing).
-
 Entropy Intuition
cc
Entropy Intuition
ccA discussion of what entropy is and what it isn't.
-
 Maxwell's Demon
cc
Maxwell's Demon
ccMaxwell's Demon: A thought experiment that seems to defy the 2nd Law of Thermodynamics
-
 More on Entropy
cc
More on Entropy
ccMore clarification as to what entropy is and what entropy is not.
-
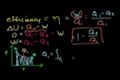 Efficiency of a Carnot Engine
cc
Efficiency of a Carnot Engine
ccDefinition of efficiency for a heat engine. Efficiency of a Carnot Engine.
-
 Carnot Efficiency 2: Reversing the Cycle
cc
Carnot Efficiency 2: Reversing the Cycle
ccSeeing how we can scale and or reverse a Carnot Engine (to make a refrigerator)
-
 Carnot Efficiency 3: Proving that it is the most efficient
cc
Carnot Efficiency 3: Proving that it is the most efficient
ccProving that a Carnot Engine is the most efficient engine
-
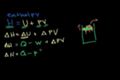 Enthalpy
cc
Enthalpy
ccUnderstanding why enthalpy can be viewed as "heat content" in a constant pressure system.
-
 Heat of Formation
cc
Heat of Formation
ccStandard heat of formation or standard enthalpy change of formation.
-
 Hess's Law and Reaction Enthalpy Change
cc
Hess's Law and Reaction Enthalpy Change
ccUsing Hess's Law and standard heats of formation to determine the enthalpy change for reactions
-
 Gibbs Free Energy and Spontaneity
cc
Gibbs Free Energy and Spontaneity
ccIntuition behind why spontaneity is driven by enthalpy, entropy and temperature. Introduction to Gibbs free energy.
-
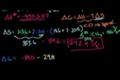 Gibbs Free Energy Example
cc
Gibbs Free Energy Example
ccDetermining if a reaction is spontaneous by calculating the change in Gibbs Free Energy.
-
 More rigorous Gibbs Free Energy/ Spontaneity Relationship
cc
More rigorous Gibbs Free Energy/ Spontaneity Relationship
ccMore formal understanding of why a negative change in Gibbs Free Energy implies a spontaneous, irreversible reaction.
-
 A look at a seductive but wrong Gibbs/Spontaneity Proof
cc
A look at a seductive but wrong Gibbs/Spontaneity Proof
ccA look at why the "proof" of the relation between changes in Gibbs Free Energy and Spontaneity is wrong in many textbooks.
-
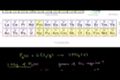 Stoichiometry Example Problem 1
cc
Stoichiometry Example Problem 1
ccFiguring grams of reactants and product produced from reaction of phosphorous and chlorine
-
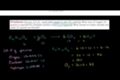 Stoichiometry Example Problem 2
cc
Stoichiometry Example Problem 2
ccStoichiometry Example Problem 2
-
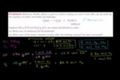 Limiting Reactant Example Problem 1
cc
Limiting Reactant Example Problem 1
ccLimiting Reactant Example Problem 1
-
 Empirical and Molecular Formulas from Stoichiometry
cc
Empirical and Molecular Formulas from Stoichiometry
ccEmpirical and Molecular Formulas from Stoichiometry
-
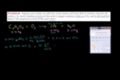 Example of Finding Reactant Empirical Formula
cc
Example of Finding Reactant Empirical Formula
ccExample of Finding Reactant Empirical Formula
-
 Stoichiometry of a Reaction in Solution
cc
Stoichiometry of a Reaction in Solution
ccStoichiometry of a Reaction in Solution
-
 Another Stoichiometry Example in a Solution
cc
Another Stoichiometry Example in a Solution
ccAnother Stoichiometry Example in a Solution
-
 Molecular and Empirical Forumlas from Percent Composition
cc
Molecular and Empirical Forumlas from Percent Composition
ccMolecular and Empirical Forumlas from Percent Composition. Example 2.9 from Kotz Chemistry book
-
 Hess's Law Example
cc
Hess's Law Example
ccHess's Law Example