Weak base
From Wikipedia, the free encyclopedia
- Acid-base extraction
- Acid-base reaction
- Acid dissociation constant
- Acidity function
- Buffer solutions
- pH
- Proton affinity
- Self-ionization of water
- Acids:
- Lewis acids
- Mineral acids
- Organic acids
- Strong acids
- Superacids
- Weak acids
- Bases:
- Lewis bases
- Organic bases
- Strong bases
- Superbases
- Non-nucleophilic bases
- Weak bases
edit
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH level compared to strong bases. Bases range from a pH of greater than 7 (7 is neutral, like pure water) to 14 (though some bases are greater than 14). The pH level has the formula:
Since bases are proton acceptors, the base receives a hydrogen ion from water, H2O, and the remaining H+ concentration in the solution determines the pH level. Weak bases will have a higher H+ concentration because they are less completely protonated than stronger bases and, therefore, more hydrogen ions remain in the solution. If you plug in a higher H+ concentration into the formula, a low pH level results. However, the pH level of bases is usually calculated using the OH- concentration to find the pOH level first. This is done because the H+ concentration is not a part of the reaction, while the OH- concentration is.
By multiplying a conjugate acid (such as NH4+) and a conjugate base (such as NH3) the following is given:
Since Kw = [H3O + ][OH − ] then, 
By taking logarithms of both sides of the equation, the following is reached:
- logKa + logKb = logKw
Finally, multipying throughout the equation by -1, the equation turns into:
- pKa + pKb = pKw = 14.00
After acquiring pOH from the previous pOH formula, pH can be calculated using the formula pH = pKw - pOH where pKw = 14.00.
Weak bases exist in chemical equilibrium much in the same way as weak acids do, with a Base Ionization Constant (Kb) (or the Base Dissociation Constant) indicating the strength of the base. For example, when ammonia is put in water, the following equilibrium is set up:
Bases that have a large Kb will ionize more completely and are thus stronger bases. As stated above, the pH of the solution depends on the H+ concentration, which is related to the OH- concentration by the Ionic Constant of water (Kw = 1.0x10-14) (See article Self-ionization of water.) A strong base has a lower H+ concentration because they are fully protonated and less hydrogen ions remain in the solution. A lower H+ concentration also means a higher OH- concentration and therefore, a larger Kb.
NaOH (s) (sodium hydroxide) is a stronger base than (CH3CH2)2NH (l) (diethylamine) which is a stronger base than NH3 (g) (ammonia). As the bases get weaker, the smaller the Kb values become. The pie-chart representation is as follows:
- purple areas represent the fraction of OH- ions formed
- red areas represent the cation remaining after ionization
- yellow areas represent dissolved but non-ionized molecules.
Contents
|
[edit] Percentage protonated
As seen above, the strength of a base depends primarily on the pH level. To help describe the strengths of weak bases, it is helpful to know the percentage protonated-the percentage of base molecules that have been protonated. A lower percentage will correspond with a lower pH level because both numbers result from the amount of protonation. A weak base is less protonated, leading to a lower pH and a lower percentage protonated.
The typical proton transfer equilibrium appears as such:
B represents the base.
In this formula, [B]initial is the initial molar concentration of the base, assuming that no protonation has occurred.
[edit] A typical pH problem
Calculate the pH and percentage protonation of a .20 M aqueous solution of pyridine, C5H5N. The Kb for C5H5N is 1.8 x 10-9.
First, write the proton transfer equilibrium:
The equilibrium table, with all concentrations in moles per liter, is
| C5H5N | C5H6N+ | OH- | |
|---|---|---|---|
| initial normality | .20 | 0 | 0 |
| change in normality | -x | +x | +x |
| equilibrium normality | .20 -x | x | x |
| Substitute the equilibrium molarities into the basicity constant |  |
| Assume that x << .20. | 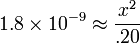 |
| Solve for x. |  |
| Check the assumption that x << .20 |  ; so the approximation is valid ; so the approximation is valid |
| Find pOH from pOH = -log [OH-] with [OH-]=x | 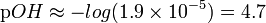 |
| From pH = pKw - pOH, |  |
| From the equation for percentage protonated with [HB+] = x and [B]initial = .20, | 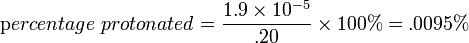 |
This means .0095% of the pyridine is in the protonated form of C5H6N+.
[edit] Examples
- Alanine, C3H5O2NH2
- Ammonia, NH3
- Methylamine, CH3NH2
- Pyridine, C5H5N
Other weak bases are essentially any bases not on the list of strong bases.

![\mbox{pH} = -\log_{10} \left[ \mbox{H}^+ \right]](http://upload.wikimedia.org/math/f/5/2/f527ad48b384c4a6f2328ab8a42a971f.png)
![\mbox{pOH} = -\log_{10} \left[ \mbox{OH}^- \right]](http://upload.wikimedia.org/math/a/c/7/ac7b52a8f87104b49e536517a397e1df.png)
![K_a \times K_b = {[H_3O^+][NH_3]\over[NH_4^+]} \times {[NH_4^+][OH^-]\over[NH_3]} = [H_3O^+][OH^-]](http://upload.wikimedia.org/math/1/4/c/14cf0b9c22142396e60185078497a046.png)
![\mathrm{K_b={[NH_4^+][OH^-]\over[NH_3]}}](http://upload.wikimedia.org/math/0/e/b/0eb8a1134449c8ec65872d591d4f306a.png)

![Percentage\ protonated = {molarity\ of\ HB^+ \over\ initial\ molarity\ of\ B} \times 100\% = {[{HB}^+]\over [B]_{initial}} {\times 100\%}](http://upload.wikimedia.org/math/4/2/9/429e42c38e12f6306fdfe7023b5bb11c.png)

![K_b=\mathrm{[C_5H_6N^+][OH^-]\over [C_5H_5N]}](http://upload.wikimedia.org/math/e/8/1/e81e22ea47d3ef3b70e324db0aa04bb5.png)