Topics
- Solutions
- Molarity
- Moles
- Volume
- Solubility
- Saturation
Description
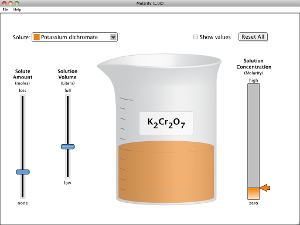
What determines the concentration of a solution? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Change solutes to compare different chemical compounds in water.
Sample Learning Goals
- Describe the relationships between volume and amount of solute to concentration.
- Explain how solution color and concentration are related.
- Calculate the concentration of solutions in units of molarity (mol/L).
- Use molarity to calculate the dilution of solutions.
- Compare solubility limits between solutes.
Version 1.02